Preparation for Samples Containing Calcium
Issue
How to prepare for samples containing Calcium
Environment
- iCAP 6000
- iCAP 7000
- iCAP Pro
- iCAP Q
- iCAP RQ
- iCAP TQ
Resolution
The following is a general guide for carbonate fusions:
- Make certain that the sample is well mixed with the sodium carbonate.
- A 5-9s pure sodium carbonate is recommended.
- Mix the sample with the flux at no more than a 1:20 ratio. Typical sample to flux ratios are in the 1:10 area.
- If organic matter is present, either the sample is mixed with the flux initially and heated slowly to 500 °C for ~ 2 hours before bring up to full temperature, or the sample can be pre-ashed at 500 °C and then the ash mixed with the flux.
- Use Pt as the crucible container material.
- Perform the fusion at 1000 °C in a muffle furnace. Avoid flames since this fusion is difficult to perform in a flame due to the high melting point of the sodium carbonate.
- Most fusions are complete in 15 minutes and some require up to 45 minutes.
- Dissolve the fuseate in dilute HCl (1:1).
Hydrolytic Stability and Preferred Matrices
- Ca begins to precipitate (as the carbonate due to dissolved CO2) from solution at a pH of between 6.7 (high conc. Ca) to 10 (low conc. Ca).
- CaO, hydroxide, carbonate, fluoride, oxalate and sulfate (dilute solutions soluble) are all insoluble in water and neutral to basic media. Precipitates are formed with neutral solutions of arsenite and arsenate as well. The most difficulty with solubility comes with the fluoride salt (very insoluble) and to a lesser extent the sulfate salt (moderately soluble) which are insoluble in acidic as well as neutral or basic media.
- Due to the presence of CO2 most solubility studies of the Ca+2 ion appear as the carbonate.
- The following table shows the improvements in the hydrolytic stability of Ca+2 with different complexing agents. The pH where precipitation of CaCO3 begins is shown for 0.1 M solutions of each complexing agent:
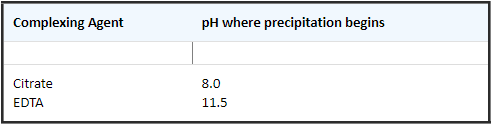
- Ca can be mixed with any of the elements at high concentrations (200 to 2000 µg/mL) except for the fluoride containing elements (Ti, Zr, Hf, Nb, Ta, W, Si, Ge, Sn, Sb, Mo). Moderate to low levels (= 100 µg/mL) can be mixed with all the elements except moderate to high levels of fluoride (F-).
Analysis
Calcium
- (Ca) is a silvery white metal resembling aluminum.
- The metal is a powerful reductant and is used to produce some metals by reduction of their oxides.
- The metal is stable in dry air but burns with incandescence in moist air.
- The metal decomposes water, evolving H2 and forming Ca (OH)2.
- The same reaction occurs more readily in acids—exercise caution.
Ca Oxide, Hydroxide, Carbonate
- Calcium oxide combines with dilute acids to form the ions of the corresponding calcium salt.
- It absorbs CO2 from the air, becoming CaCO3. In moist air CaO becomes Ca(OH)2, the reaction taking place rapidly, with increase in volume and generation of significant heat if sufficient water is present.
- The Ca(OH)2 dissolves readily in dilute acids and is much less soluble in water than the corresponding Ba and Sr hydroxides.
NOTE: The oxide, hydroxide and carbonate are all readily soluble in dilute acids. Most analysts prefer dilute (1:1) nitric acid.
Minerals and Ores
- Calcium occurs combined as carbonate, sulfate, phosphate, fluoride, silicate, and in a large number of complex compounds associated with a number of elements, among which are silicon, iron, aluminum, boron, titanium, sodium and potassium.
- Calcium is found in all mineral springs, artesian and river water, primarily as bicarbonate.
- As oxalate, it occurs in plants, as phosphate in bones, and is an essential constituent of many rock-forming minerals.
In addition...
- Since the Ca minerals contain such a wide assortment of other elements, it is considered best to prepare Ca containing minerals/ores by fusion with either lithium carbonate in graphite crucibles or sodium carbonate in Pt crucibles. The fuseate is dissolved in dilute HCl.
- When Ca is associated with fluoride as CaF2 (fluorite or fluor spar), the carbonate fusion will not work and it is suggested that the analyst fuse the sample with H3BO3 or B2O3 . This fusion is carried out in Pt crucibles at 1000 °C where the sample to flux ratio is at least 1:8 and can be as high as 1:30. The CaF2 is opened out by this fusion procedure and the fluoride is tied up with the boron making the dissolution of the fuseate in water or dilute acid possible without reformation of the CaF2.
- Lithium tetraborate (Li2B4O7) has proven to be a very useful way of opening out any of the minerals associated with Ca including the fluorite. This fusion is carried out in Pt for ~ 10 minutes at 1000 °C and the fuseate is dissolved at room temperature by stirring with dilute (5 % v/v) nitric acid. When metal ions are present that are readily hydrolyzed, dissolution in the presence of EDTA in 0.01 M HCl is advantageous.
Alloys
- Ca metal is not used to a great extent nor are its alloys. Although commercial alloys are relatively rare, their dissolution can be affected using dilute nitric or nitric/HCl mixtures.
Organic Matrices
- This includes a wide variety of materials including oil additives, petroleum matrices, coal, organic plant material, biological material, synthetic organics, and more. Samples can be digested with nitric/perchloric.
- It is also very acceptable to dry ash organic samples for Ca analysis in a Pt crucible and then bring the resulting CaO into solution using a sodium carbonate fusion. Or if Ca alone is sought, dissolution in dilute nitric or HCl.
Attachment(s)
| File | Last Modified |
|---|---|
| Image - Ca - pH where precipitation begins.png | August 02, 2022 |

