Preparation for Samples Containing Uranium and Thorium
Issue
How to prepare for samples containing Uranium and Thorium
Environment
- iCAP 6000
- iCAP 7000
- iCAP Pro
- iCAP Q
- iCAP RQ
- iCAP TQ
Resolution
The following is a general guide for carbonate fusions:
- Make certain that the sample is well mixed with the sodium carbonate.
- A 5-9s pure sodium carbonate is recommended.
- Mix the sample with the flux at no more than a 1:20 ratio. Typical sample to flux ratios are in the 1:10 area.
- If organic matter is present, either the sample is mixed with the flux initially and heated slowly to 500 °C for ~ 2 hours before bring up to full temperature, or the sample can be pre-ashed at 500 °C and then the ash mixed with the flux.
- Use Pt as the crucible container material.
- Perform the fusion at 1000 °C in a muffle furnace. Avoid flames since this fusion is difficult to perform in a flame due to the high melting point of the sodium carbonate.
- Most fusions are complete in 15 minutes and some require up to 45 minutes.
- Dissolve the fuseate in dilute HCl (1:1).
Hydrolytic Stability and Preferred Matrices
- The Th+4 ion is most resistant to hydrolysis of all the quadrivalent ions because it is the largest.
- Th(OH)3+ and Th(OH)22+ become detectable at pH 2-3 and precipitation of the hydroxide begins at pH 3.5 to 4.0.
- Of the common ions the presence of acetate and sulfate increase the pH at which thorium hydroxide precipitation begins thereby improving the hydrolytic stability.
- Th+4 should not be mixed with oxalate, phosphate, sulfate, or fluoride where insoluble precipitates are formed.
- U(IV) begins precipitation as the hydroxide at a pH of about 1.5. The presence of sulfate improves this pH to 2.5.
- UO2+2 begins to precipitate as UO2 (OH)2 * H2O at a pH of 3.2. Acetate, fluoride and sulfate all improve the hydrolytic stability with fluoride giving the most striking improvement to a pH of 7.
- Both the IV and uranyl forms of U prefer a nitric acid media. If unsure of the oxidation state, then avoid fluoride, phosphate, and sulfate.
- Nitrate solutions of U and Th are stable with any of the elements that are stable in nitric acid matrices. Avoid elements containing HF and HCl in the matrix, as well as P as the orthophosphate and S as the sulfate. The pH of all mixtures should be less than 2 for long-term stability.
- The elements that are stable/soluble and commonly diluted in aqueous/HNO3 are shaded in red below:
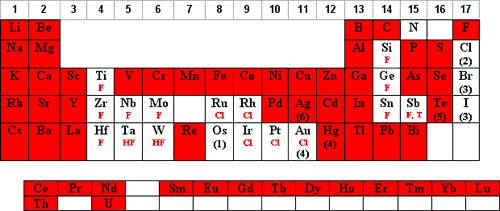
- Os should never be mixed with HNO3 due to the formation of the very volatile OsO4 Shape.
- Cl is oxidized to molecular Cl2 which is volatile and adsorbs on plastic.
- Br and I are oxidized to molecular Br2 and I2 which adsorb onto plastic.
- Dilutions of Hg and Au in HNO3 below 100 ppm should be stored in borosilicate glass due to Hg+2 adsorption on plastic.
- Not soluble above concentrations of 1000 µg/mL.
- Trace levels of HCl or Cl- will form AgClShape, which will photoreduce to Ag0.
- F Denotes that the element can be diluted in HNO3 if complexed with F-.
- Cl Denotes that the element can be diluted in HNO3 if complexed with Cl-.
- HF Denotes that the element should have excess HF present when diluted with HNO3.
- T Denotes that the tartaric acid complex can be diluted in HNO3.
Analysis
Overview
- Uranium (U) and Thorium (Th) are considered "Rare Earth" (RE) elements.
- More specifically, Th and U are members of the actinide series, which are elements following actinium, i.e., starting with atomic number 90. Although considered RE elements, Th and U have a chemistry that does not closely resemble the lanthanides.
- Rather, these two elements have a rather unique chemistry and cannot be said to closely resemble any other particular group of elements.
- In some respects, they behave like the RE elements and in others their chemistry is more like that of Hf, Ta, and W.
The Metals
- Th: The metal dissolves readily in Aqua Regia and is soluble in HCl (standard reduction potential is -1.90 volts vs N.H.E), but is not readily soluble in other non-oxidizing acids such as sulfuric acid or hydrofluoric acid.
- Nitric acid alone passivates the surface rendering it insoluble.
- The metal is not attacked by the alkali hydroxides.
NOTE: U: The reduction potential is close (-1.80 volts vs N.H.E) to that of Th and so is the solubility of the metal. Metallic uranium is soluble in HCl or HNO3 and soluble to some extent in cold dilute sulfuric acid. The caustic alkalis have no apparent action.
Oxides, Hydroxides, and Salts
- Th: The principal oxide of thorium is ThO2, which is insoluble in acids except hot, concentrated sulfuric acid.
- It is not affected by fusion with the alkalis.
- Thorium hydroxide, when freshly precipitated, is readily soluble in acids, but after drying is more resistant.
- It is soluble in the alkali carbonates.
- Thorium chloride and nitrate are soluble in water.
- The anhydrous sulfate is soluble in ice water, but upon heating, it separates as a hydrate.
- The solution chemistry of Th is centered on the chemistry of Th+4. Th+4 forms an insoluble hydroxide with NaOH or NH4OH.
- The precipitate is not soluble in an excess of the reagent.
- Alkali carbonates will precipitate Th+4 as a basic carbonate, which is readily soluble in an excess of the concentrated reagent.
- Th+4 should not be mixed with oxalate, phosphate, sulfate, or fluoride where insoluble precipitates are formed.
- U: UO2 is soluble in nitric acid and aqua regia and will dissolve with difficulty in HCl, HBr, and sulfuric acid.
- U3O8 is insoluble in water, readily soluble in nitric acid, but only slightly soluble in HCl.
- In general, uranates (UO4-2) are insoluble in water and soluble in acids.
- Most uranyl (UO2+2) salts are soluble in water and those insoluble are dissolved in HCl.
- The salts of uranium may be divided chiefly into two classes:
- (1) the uranous or uranium IV, U+2 and
- (2) the uranyl UO2++.
- Fluoride will precipitate the IV, but not the uranyl ion. However, to play it safe avoid HF as well as phosphate, sulfate, and carbonate.
Minerals
- Thorium-bearing minerals are widespread, but generally in small amounts.
- Monazite is the principal source of Th.
- Uranium is estimated to be present in the earth's crust at about 4 ppm and occurs combined with silicates, phosphates, and zirconates.
- The use of lithium tetraborate (Li2B4O7) has proven to be a very useful way of opening out minerals associated with the rare earths, including Th and U. A procedure reported by Knaack, C., Cornelius, S.B. and Hooper, P.R., GeoAnalytical Lab, Washington State university, December, 1994 involves fusion of the sample (2 grams) with an equal amount of the lithium tetraborate flux in a carbon crucible at 1000 °C in a muffle furnace for 30 minutes.
- The fuseate is then treated with HF, nitric, and then perchloric acids with a repetition of the perchloric acid treatment to dissolve fluorides.
- The Final solution is made to 60 mL and contains nitric acid, hydrogen peroxide, and HF, where In, Re, and Ru are added as internal standards for an ICP-MS measurement.
- This method is reported to be applicable for the lanthanides (La thru Lu) together with Ba, Rb, Y, Nb, Cs, Hf, Ta, Pb, Th, U, Sr, and Zr.
Catalysts
Zeolites and to a lesser extent silica gel are common supports for the REEs.
The following procedure may prove useful:
- Weigh 0.1 to 0.5 grams of sample into a Pt crucible or dish.
- Add 20 mL of HClO4 and 20 to 50 mL of HF depending upon sample size.
- Heat on a hot plate to the dense white fumes of HClO4.
- Cool and add 15 mL of a 4% H3BO3 solution.
- Quantitatively transfer to a quartz or Vycor beaker and heat to heavy dense fumes.
- Transfer to a volumetric flask (250 to 1000 depending), add dilute with DI water and HCl to 10% v/v HCl.
NOTE: The above procedure is presented only as a guide into the basic theory behind the acid digestion of silica containing catalysts where HF is used to break apart and dissolve the entire zeolites, heating to fumes of HClO4 then ridding the sample of excess HF, and very importantly, the addition of boric acid and heating to fumes results in the dissolution of any insoluble RE fluorides. Si is lost during the preparation.
Organic Matrices
- Samples can be digested with nitric/perchloric.
- It is also very acceptable to dry ash organic samples for rare earth (RE) element analysis in a Pt crucible and then bring the resulting RE oxides into solution using a sodium carbonate fusion, or if the REE alone is sought, dissolution in dilute nitric or HCl.
Attachment(s)
| File | Last Modified |
|---|---|
| Image - Ba, Sr - pH where precipitation begins.png | August 02, 2022 |
| Image - Ba, Sr vs Ca.png | August 02, 2022 |
| Image - Periodic Table.gif | August 02, 2022 |

