Preparation for Samples Containing Barium and Strontium
Issue
How to prepare for samples containing Barium and Strontium
Environment
- iCAP 6000
- iCAP 7000
- iCAP Pro
- iCAP Q
- iCAP RQ
- iCAP TQ
Resolution
The following is a general guide for sodium carbonate fusions:
- Make certain that the sample is well mixed with the sodium carbonate.
- A 5-9s pure sodium carbonate is recommended.
- Mix the sample with the flux at no more than a 1:20 ratio. Typical sample to flux ratios are in the 1:10 area.
- If organic matter is present either the sample is mixed with the flux initially and heated slowly to 500 deg C for ~2 hours before bring up to full temperature or the sample can be pre-ashed at 500 °C and then the ash mixed with the flux.
- Use Pt as the crucible container material.
- Perform the fusion at 1000 °C. in a muffle furnace. Avoid flames since this fusion is difficult to perform in a flame due to the high melting point of the sodium carbonate.
- Most fusions are complete in 15 minutes and some require up to 45 minutes.
- Dissolve the fuseate in dilute HCl (1:1).
- When Ba is associated with sulfate as BaSO4 or the fluoride, it is suggested that the analyst fuse the sample with H3BO3 or B2O3
- This fusion is carried out in Pt crucibles at 1000 °C where the sample to flux ratio is at least 1:8 and can be as high as 1:30. The SO3 is expelled due to the high temperature of the acidic melt.
- The MF2 is opened out by this fusion procedure and the fluoride is tied up with the boron, making the dissolution of the fuseate in water or dilute acid possible without reformation of the MF2.
Lithium tetraborate
- (Li2B4O7) has also proven to be a very useful way of opening out many of the ores associated with Ba and Sr.
- This fusion is carried out in Pt for ~10 minutes at 1000 °C and the fuseate is dissolved at room temperature by stirring with dilute (5% v/v) nitric acid.
- When metal ions that are readily hydrolyzed are present, dissolution in the presence of EDTA in 0.01 M HCl is advantageous.
Alloys
- The Ba and Sr metals are not used to a great extent nor are their alloys.
- Although commercial alloys are relatively rare, their dissolution can be affected using dilute nitric or nitric/HCl mixtures.
Organic Matrices
- These include a wide variety of materials including oil additives (Ba), petroleum matrices, coal, organic plant material, biological material, synthetic organics, etc.
- Samples can be digested with nitric/perchloric.
NOTE: It is also very acceptable to dry ash organic samples for Ba or Sr analysis in a Pt crucible and then bring the resulting oxides into solution using a sodium carbonate fusion or if Ba or Sr alone are sought, dissolution in dilute nitric or HCl.
Hydrolytic Stability and Preferred Matrices
- Ba and Sr begin to precipitate (as the carbonate due to dissolved CO2) from solution at a pH of between 6.2 (high conc.) to 10 (low conc.).
- Following are solubility data for Sr and Ba as compared to Ca (g/100 mL H2O):
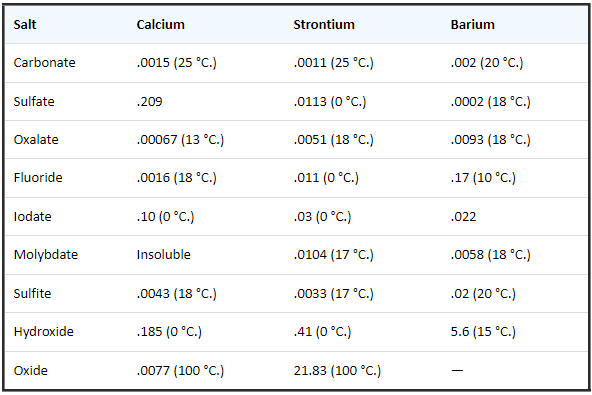
- The following table shows the improvements in the hydrolytic stability of Ba+2 and Sr+2 with different complexing agents. The pH where precipitation of BaCO3 and SrCO3 begins is shown for 0.1 M solutions of each complexing agent:

- Ba and Sr can be mixed with any of the elements at high concentrations (200 to 2000 µg/mL) with the exception of the fluoride containing elements (Ti, Zr, Hf, Nb, Ta, W, Si, Ge, Sn, Sb, Mo) and hexavalent chrome and sulfate in the case of Ba.
- Moderate to low levels (≤ 100 µg/mL) can be mixed with all of the elements except moderate to high levels of fluoride (F-) and sulfate (Ba only).
Analysis
- Both barium (Ba) and strontium (Sr) are silvery white metals resembling aluminum.
- The metals are unstable in air and burn with incandescence in moist air or when heated.
- They decompose water, evolving H2 and forming Sr(OH)2 or Ba(OH)2. The same reaction occurs more readily in acids—exercise caution.
Oxides, Hydroxides, Carbonates
- The oxides are white and are obtained by ignition of the hydroxide, carbonate, nitrate, oxalate and all their organic salts.
- The oxides, hydroxides and carbonates are all readily soluble in dilute acids combining to form the ions of the corresponding salt.
- They absorb CO2 from the air, becoming carbonates.
- In moist air, oxides become hydroxides, with the generation of heat if sufficient water is present.
- The hydroxides dissolve readily in dilute acids and are much more soluble in water than the corresponding Ca hydroxide.
NOTE: The oxide, hydroxide, and carbonate are all readily soluble in dilute acids. Most analysts prefer dilute (1:1) nitric acid.
Minerals and Ores
- Since the ores contain such a wide assortment of other elements it is considered best to prepare them by fusion with either lithium carbonate in graphite crucibles or sodium carbonate in Pt crucibles.
- If barium sulfate is present, the fusion converts it to the barium carbonate and the corresponding sodium sulfate, which can be leached out from the fuseate with water prior to dissolution of the fuseate in dilute HCl.
Attachment(s)
| File | Last Modified |
|---|---|
| Image - Ba, Sr vs Ca.png | August 02, 2022 |
| Image - Ba, Sr - pH where precipitation begins.png | August 02, 2022 |

