Preparation for Samples Containing Beryllium
Issue
How to prepare for samples containing Beryllium
Environment
- iCAP 6000
- iCAP 7000
- iCAP Pro
- iCAP Q
- iCAP RQ
- iCAP TQ
Resolution
The sulfuric peroxide procedure is as follows:
- Add a 0.1 gram sample to a 125 mL Erlenmeyer flask.
- Add 5 milliliters of 98% sulfuric acid.
- Heat until dense white fumes form.
- Sample will be dark in color.
- Add 30% hydrogen peroxide drop-wise until solution clears.
- Continue heating until solution remains clear with dense white fumes and does not revert back to a dark color.
- Remove from heat.
- Cool and dilute to desired volume with 18 megohm water.
- It is also very acceptable to dry ash organic samples for Be analysis in a Pt crucible and then bring the resulting BeO into solution with one of the acids or fusion suggested above.
- It is important to keep the ashing temperature at 450 °C.
- The high temperature-ignited BeO is very difficult to dissolve with acids.
- Hydrolytic Stability and Preferred Matrices Be has a tendency to hydrolyze in water at a pH of ≥ 4.2.
- When diluting Be standards they should only be added to pre-acidified water.
- Be(OH)2 is one of the least soluble Be compounds.
- It is precipitated as the hydroxide by NH4OH or the fixed alkalis.
- In addition, beryllium hydroxide has a tendency to form colloidal suspensions that are difficult to detect visually.
- There are three forms of the hydroxide, namely, the freshly precipitated amorphous form (readily soluble in dilute acid), the a-form produced on standing and the ß-form produced after prolonged heating.
- Stability constants for the a-form are most readily available.
- The following table shows the improvements in hydrolytic stability of Be with different complexing agents.
- The pH where precipitation begins is shown for 0.1 M solutions of each complexing agent:
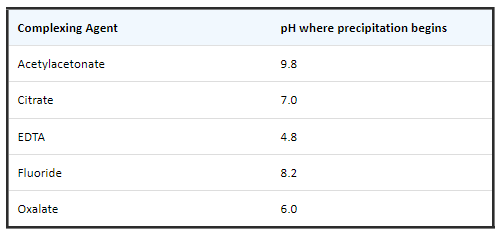
- Be can be mixed with any of the elements, at moderate to high concentrations (< 1 to 1000 µg/mL) but care must be taken to maintain a pH < 2 when nitric acid is used. One of the above complexing agents is needed for solutions with a pH > 2.
Analysis
- Beryllium (Be) is the only stable light metal of high melting point.
- Be metal is not affected by water or cold nitric acid.
- It's readily soluble in NaOH, HCl, hot nitric acid and in dilute sulfuric acid.
- BeO Beryllium oxide finds useful applications in refractory crucibles because of its high melting point (2570 °C) and good resistance to thermal shock.
- BeO is insoluble in dilute HCl, but can be put into solution by boiling with nitric, HF or sulfuric acids, or by fusion with potassium bisulfate and dissolution of the fuseate in acidified water.
- Minerals Beryl (3BeO-Al2O3-6SiO2) is the only mineral of more than thirty known that is of any commercial importance.
- Powdered beryl ores are commonly fused with sodium carbonate with the proportion by weight of sodium carbonate to ore at two parts to one.
- A sodium carbonate fusion is performed in Pt at 1000 °C and the ore requires the full temperature for 30-45 minutes.
- The fuseate is brought into solution with dilute HCl, nitric or combinations with HF depending upon the dilution ratio of sample and length of time standing before analysis (keep the concentration of Si below or at ~10 ppm for long term solution stability without HF present; otherwise add HF to maintain long term stability of the Si).
- Beryl is also soluble in HF/sulfuric acid mixtures.
- Alloys Be-Cu alloys are famous for their excellent properties.
- These include master alloy (4.00 -4.25% Be with Fe, Al and Si as impurities) and the ternary alloys, employing Ni or Co hardener, made from master alloy.
- Alloys of Be with Cu or nickel are typically dissolved with nitric acid while alloys with Fe, Al or Mg are dissolved with HCl.
Attachment(s)
| File | Last Modified |
|---|---|
| Image - Be - pH where precipitation begins.png | August 02, 2022 |

